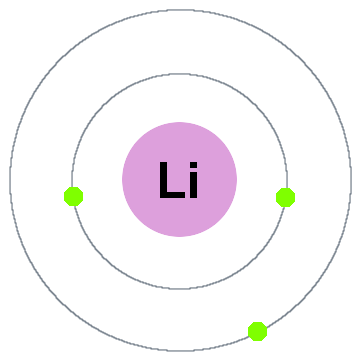
That means there are 3 electrons in a lithium atom. Looking at the picture, you can see there are two electrons in shell one and only one in shell two. More about the history and places to find lithium. 4.2/5 (1,717 Views. 34 Votes) However, a lithium atom is neutral because there are 3 negative electrons outside the nucleus. A neutral lithium atom will also have 3 electrons. The negative electrons balance the charge of the positive protons in the nucleus. The Fermi energy for lithium is 4.72 eV at T = 0. Calculate the number of conduction electrons per unit volume in lithium. How many electrons per atom is this? The density of lithium is 535 kg/m^3.
By Prof. L. Kaliambos (Natural Philosopher in New Energy)
October 19, 2015
Lithium is an atom of the chemical element Lithium with symbol Li and atomic number 3. However unlike for hydrogen atom , a closed-form solution to the Schrödinger equation for the many-electron atoms like the lithium atom has not been found. So, under the invalid relativity (EXPERIMENTS REJECT RELATIVITY) various approximations, such as the Hartree–Fock method, could be used to estimate the ground state energies. Under these difficulties I published my paper 'Spin-spin interactions of electrons and also of nucleons create atomic molecular end nuclear structures' (2008) by analysing carefully the electromagnetic interactions of two spinning electrons of opposite spin which give a simple formula for the solution of such ground state energies. Hence today it is well known that the correct electron configuration of Lithium should be given by this correct image including the following electron configuration: 1s22s1
According to the “Ionization energies of the elements-WIKIPEDIA” we observethat E1 = 5.39 eV, E2 = 75.6 eV, and E3 =122.4 eV. For the explanation of the first ionization energy (E1 =5.39 eV) due to the outer electron (2s1)with n = 2 you can see my paper “Explanation of lithium ionizations”.
Whereas for calculating thesummation of the second and third ionization energies
E = (75.6 +122.4) = 198 eV
which gives the groundstate energy of Lithium (Z = 3) you can see my paper published in Ind. J. Th.Phys. (2008) entitled “ Spin-spin interactions of electrons and also ofnucleons create atomic molecular and nuclear structures”. (See it in “User Kaliambos”). In that paper I showed that the ground state energy (-E = - 198eV) is the binding energy of the twospinning electrons of opposite spin of the Li+ with n = 1 whenZ = 3. That is
-E = - 198 = (-27.21)Z2 +(16.95)Z - 4.1
Templates for ms powerpoint presentations microsoft. Since Z = 3 one gets
-E = (-27.2)32 + (16.95)3 - 4.1 = -122.4 + 244.8 - 50.84 + 4.1 = -198 eV
Historically, despite theenormous success of the Bohr model and the quantum mechanics of the Schrodingerequation based on the well-established laws of electromagnetism in explainingthe principal features of the hydrogen spectrum and of other one-electronatomic systems, so far, under the abandonment of natural laws neither was ableto provide a satisfactory explanation of the two-electron atoms. In atomicphysics a two-electron atom is a quantum mechanical system consisting of onenucleus with a charge Ze and just two electrons. This is the first case ofmany-electron systems. The first few two-electron atoms are:
Z =1 : H- hydrogenanion. Z = 2 : He helium atom. Z = 3 : Li+ lithiumatom anion. Z = 4 : Be2+ beryllium ion. Z =5 : B3+ boron.
Prior to the development ofquantum mechanics, an atom with many electrons was portrayed like the solarsystem, with the electrons representing the planets circulating about thenuclear “sun”. In the solar system, the gravitational interaction betweenplanets is quite small compared with that between any planet and the verymassive sun; interplanetary interactions can, therefore, be treated as smallperturbations.
However, In the helium atomwith two electrons, the interaction energy between the two spinning electronsand between an electron and the nucleus are almost of the same magnitude, and aperturbation approach is inapplicable.
In 1925 the two young Dutchphysicists Uhlenbeck and Goudsmit discovered the electron spin according towhich the peripheral velocity of a spinning electron is greater than the speedof light. Since this discovery invalidates Einstein’s relativity it met muchopposition by physicists including Pauli. Under the influence of Einstein’sinvalid relativity physicists believed that in nature cannot existvelocities faster than the speed of light.(See my FASTER THAN LIGHT).
So great physicists likePauli, Heisenberg, and Dirac abandoned the natural laws of electromagnetism infavor of wrong theories including qualitative approaches under an idea ofsymmetry properties between the two electrons of opposite spin which lead tomany complications. Thus, in the “Helium atom-Wikipedia” one reads: “Unlike forhydrogen a closed form solution to the Schrodinger equation for the helium atomhas not been found. However various approximations such as the Hartree-Fockmethod ,can be used to estimate the ground state energy and wave function ofatoms”.
It is of interest to notethat in 1993 in Olympia of Greece I presented at the international conference“Frontiers of fundamental physics” my paper “Impact of Maxwell’s equation of displacement current on electromagnetic laws and comparison of the Maxwellian waves with our model of dipolic particles '. The conference was organised by the natural philosophers M. Barone and F. Selleri, who gave me an award including a disc of the atomic philosopher Democritus, because in that conference I showedthat LAWS AND EXPERIMENTS INVALIDATE FIELDS AND RELATIVITY .At the same time I tried to find not only the nuclear force and structurebut also the coupling of two electrons under the application of the abandonedelectromagnetic laws. For example in the well known photoelectric effect theabsorption of light contributed not only to the increase of the electron energybut also to the increase of the electron mass because the particles of lighthave mass m = hν/c2 .(See my DISCOVERY OF PHOTON MASS ).
However the electron spinwhich gives a peripheral velocity greater than the speed of light cannot beaffected by the photon absorption. Under this condition the electromagnetic force can be written as
Fem = Fe - Fm .
Therefore in my researchthe integration for calculating the mutual Fem led to thefollowing relation:
Fem = Fe - Fm = Ke2/r2 - (Ke2/r4)(9h2/16π2m2c2)
Of course for Fe =Fm one gets the equilibrium separation ro =3h/4πmc = 578.8/1015 m.
That is, for r <578.8/1015 m the two electrons of opposite spin exert anattractive electromagnetic force, because the attractive Fm isstronger than the repulsive Fe . Here Fm isa spin-dependent force of short range. As a consequence this situationprovides the physical basis for understanding the pairing of two electronsdescribed qualitatively by the Pauli principle, which cannot be applied in thesimplest case of the deuteron in nuclear physics, because the binding energybetween the two spinning nucleons occurs when the spin is not opposite (S=0)but parallel (S=1). According to the experiments in the case of two electronswith antiparallel spin the presence of a very strong external magnetic fieldgives parallel spin (S=1) with electric and magnetic repulsions given by

Fem = Fe + Fm
So, according to thewell-established laws of electromagnetism after a detailed analysis of paired electrons in two-electron atoms I concluded that at r < 578.8/1015 m a motional EMF produces vibrations ofpaired electrons.
Unfortunately today manyphysicists in the absence of a detailed knowledge believe that the twoelectrons of two-electron atoms under the Coulomb repulsion between theelectrons move not together as one particle but as separated particlespossessing the two opposite points of the diameter of the orbit aroundthe nucleus. In fact, the two electrons of opposite spin behave like oneparticle circulating about the nucleus under the rules of quantum mechanicsforming two-electron orbitals in helium, beryllium etc. In my paper of 2008, I showed that the positive vibration energy (Ev) described in eV dependson the Ze charge of nucleus as
Ev = (16.95)Z -4.1
Of course in the absence ofsuch a vibration energy Ev it is well-known that the ground state energyE described in eV for two orbiting electrons could be given by the Bohr modelas
E = (-27.2) Z2.
So the combination of theenergies of the Bohr model and the vibration energies due to the opposite spinof two electrons led to my discovery of the ground state energy of two-electronatoms given by
E = (-27.2) Z2 +(16.95 )Z - 4.1
For example the laboratorymeasurement of the ionization energy of H- yields an energy ofthe ground state E = - 14.35 eV. In this case since Z = 1 weget E -27.2 + 16.95 - 4.1 = -14.35 eV. In the same waywriting for the helium Z = 2 we get

E = - 108.8 + 32.9 - 4.1 =-79.0 eV
which is equal to thelaboratory measurement. In the same way we can calculate the ground stateenergies for the Z = 3 : Li+ ion.
The discovery of thissimple formula based on the well-established laws of electromagnetism was thefirst fundamental equation for understanding the energies of many-electronatoms, while various theories based on qualitative symmetry properties lead tocomplications. For example in “Lithium atom-WIKIPEDIA” we read: “Similarly to the case of the helium atom, aclosed-form solution to the Schrödinger equation for the lithium atom has notbeen found. However, various approximations, such as the Hartree–Fock method,can be used to estimate the ground state energy and wavefunction of the atom.Quantum defect is a value that describes the deviation from hydrogenic energylevels.”
Element Lithium - Li
Comprehensive data on the chemical element Lithium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Lithium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Lithium Menu
- Lithium Page One
- Lithium Page Two
- Lithium Page Three
Overview of Lithium
- Atomic Number: 3
- Group: 1
- Period: 2
- Series: Alkali Metals
Lithium's Name in Other Languages
- Latin: Lithium
- Czech: Lithium
- Croatian: Litij
- French: Lithium
- German: Lithium - s
- Italian: Litio
- Norwegian: Litium
- Portuguese: Litio
- Russian: Литий
- Spanish: Lítio
- Swedish: Litium
Atomic Structure of Lithium
- Atomic Radius: 2.05Å
- Atomic Volume: 13.1cm3/mol
- Covalent Radius: 1.23Å
- Cross Section (Thermal Neutron Capture) σa/barns: 70.5
- Crystal Structure: Cubic body centered
- Electron Configuration:
- 1s2 2s1
- Electrons per Energy Level: 2,1
- Shell Model
- Shell Model
- Ionic Radius: 0.76Å
- Filling Orbital: 2s1
- Number of Electrons (with no charge): 3
- Number of Neutrons (most common/stable nuclide): 4
- Number of Protons: 3
- Oxidation States: 1
- Valence Electrons: 2s1
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Lithium
- Electrochemical Equivalent: 0.259g/amp-hr
- Electron Work Function: 2.9eV
- Electronegativity: 0.98 (Pauling); 0.97 (Allrod Rochow)
- Heat of Fusion: 3kJ/mol
- Incompatibilities:
- water, acids, oxidizing agents
- Ionization Potential
- First: 5.392
- Second: 76.638
- Third: 122.451
- Valence Electron Potential (-eV): 19
Physical Properties of Lithium
- Atomic Mass Average: 6.941
- Boiling Point: 1615.15K 1342°C 2448°F
- Coefficient of lineal thermal expansion/K-1: 56E-6
- Conductivity
- Electrical: 0.108 106/cm Ω
Thermal: 0.847 W/cmK
- Electrical: 0.108 106/cm Ω
- Density: 0.534g/cc @ 300K
- Description:
- Soft silvery-white metal. Lightest of metals.
- Elastic Modulus:
- Bulk: 11/GPa
- Rigidity: 4.24/GPa
- Youngs: 4.91/GPa
- Enthalpy of Atomization: 160.7 kJ/mole @ 25°C
- Enthalpy of Fusion: 3 kJ/mole
- Enthalpy of Vaporization: 134.7 kJ/mole
- Flammablity Class: Flammable solid
- Freezing Point:see melting point
- Hardness Scale
- Mohs: 0.6
- Heat of Vaporization: 145.92kJ/mol
- Melting Point: 453.85K 180.7°C 357.3°F
- Molar Volume: 13 cm3/mole
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 3.6J/gK
- Vapor Pressure = 1.63E-08Pa@180.7°C
Regulatory / Health
- CAS Number
- 7439-93-2
- UN/NA ID and ERG Guide Number
- UN1415 / 138
- RTECS: OJ5540000
- OSHAPermissible Exposure Limit (PEL)
- No limits set by OSHA
- OSHA PEL Vacated 1989
- No limits set by OSHA
- NIOSHRecommended Exposure Limit (REL)
- No limits set by NIOSH
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 0.004
- Bone/p.p.m: 1.3
- Liver/p.p.m: 0.025
- Muscle/p.p.m: 0.023
- Daily Dietary Intake: 0.1-2 mg
- Total Mass In Avg. 70kg human: 7 mg
Who / Where / When / How
- Discoverer: Johann A. Arfvedson
- Discovery Location: Stockholm Sweden
- Discovery Year: 1817
- Name Origin:
- Greek: lithos (stone)
- Abundance of Lithium:
- Earth's Crust/p.p.m.: 20
- Seawater/p.p.m.: 0.17
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 10
- Sources of Lithium:
- Spodumene, ambylgonite, lepidolite and desert lake brines. Also obtained by passing electric charge through melted lithium chloride. Around 39,000 tons of lithium is produced each year. The primary source of lithium is the USA.
- Uses of Lithium:
- Used in batteries, ceramics, glass, lubricants, alloy hardeners, pharmaceuticals, hydrogenating agents, heat transfer liquids, rocket propellants, vitamin A synthesis, nuclear reactor coolant, underwater buoyancy devices and the production of tritium. Deoxidizer in copper and copper alloys.
- Additional Notes:
- Lithium was first isolated in 1821 by W.T Brande. Near its melting point, lithium ignites in air. Lithium posses a dangerous fire and explosion risk when exposed to water, acids or oxidizing agents. It reacts exothermally with nitrogen in moist air at high temperatures. In solution lithium is toxic and targets the central nervous system.
Lithium Menu
- Lithium Page One
- Lithium Page Two
- Lithium Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Lithium - Li. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Li.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Li.html'>echo Periodic Table of Elements: Lithium - Li (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Lithium - Li is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
Number Of Electrons In Lithium Valence Shell
PLEASE, if you like an article we published simply link to it on our website do not republish it.

